Abstract
The upstream binding transcription factor (UBTF) is a nucleolar protein that regulates the transcription of ribosomal genes as a component of the RNA Pol I pre-initiation complex. Recently, tandem duplication mutations of the UBTF gene (UBTF-TDs) were identified as recurrent alterations in pediatric AML (Umeda et al., 2022), potentially representing a new subtype-defining lesion. UBTF-TDs were found to be associated with normal karyotype (NK) or trisomy 8 (+8), FLT3-ITD and WT1 mutations as well as poor prognosis. Data on the role of UBTF-TD in adult AML are limited. In the present study, we therefore analyzed the prevalence and prognostic significance of UBTF-TDs in a large cohort of adult AML patients (pts).
Methods: Pts included were registered and treated in clinical protocols of the Study Alliance Leukemia (SAL) (AML96, AML2003, AML60+, SORAML) or the SAL-registry.
Screening for UBTF-TDs was done by PCR covering exon 13 of the UBTF gene with 6-FAM labelled primers followed by high-resolution fragment analysis using genomic DNA isolated at diagnosis. Mutations were confirmed by Sanger sequencing. All UBTF mutant samples were further analyzed by targeted next generation sequencing (Archer Myeloid Panel) for the presence of co-occurring alterations.
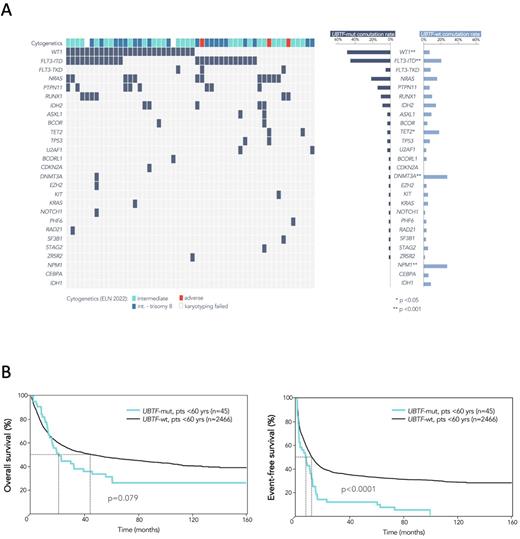
Results: Somatic mutations in UBTF were identified in 52 (1.2%) of 4247 pts. Mutations varied in size (3 to 309 bps), all yielding in-frame insertions, duplications or deletions in exon 13 of the UBTF gene. UBTF-TD AMLs frequently presented with a normal karyotype (46%) or +8 (36.5%). Similar to findings in pediatric pts, the co-mutation profile showed significant differences between UBTF-mut and UBTF-wt pts, i.e. UBTF-TDs were significantly associated with FLT3-ITD (48%, p<.001) or WT1 mutations (52%, p<.001), while DTA co-mutations were exceedingly rare (DNMT3A 1.9%; TET2 3.8%; ASXL1 3.8%) (Fig. 1A). Furthermore, UBTF-TDs and several class-defining lesions, i.e. reciprocal translocations such as RUNX1::RUNX1T1,DEK::NUP214 or mutations in CEBPA or NPM1 were mutually exclusive.
The prevalence of UBTF-TDs showed a strong negative correlation with increasing age, ranging from 10% in patients <20 years (yrs) to 0.05% in patients >70 yrs, respectively. Accordingly, pts with UBTF-TDs were significantly younger (median age 41 yrs; range 17-77 yrs) than wt pts (med. 57 yrs; range 15-90 yrs, p<.001).
Association with clinical parameters revealed significantly lower hemoglobin levels and platelet counts at diagnosis for UBTF-mut pts compared to UBTF-wt pts (median Hb 8.7 vs. 9.2 g/dL; p=.02; median PLT 31.5 vs 53 x 109/L; p=.003). Although UBTF-TDs mainly affected younger pts, cytomorphologic assessment revealed a clear association with myelodysplastic changes in about 60% of patients.
Univariate analysis showed a significantly higher rate of complete remissions (CR) in UBTF-mut pts (92.3%) compared to UBTF-wt pts (70.8%) (p<.001). However, UBTF-mut pts had shorter overall (OS) and event-free survival (EFS). This effect was particularly pronounced in the subgroup of pts under 60 years of age (median OS 20.4 months in UBTF-mut pts vs. 45.3 months in UBTF-wt pts, p=.079; median EFS 6.7 months in UBTF-mut pts vs. 11 months in UBTF-wt pts, p<.001) (Fig.1B), who represented the majority (45/52, 87%) of UBTF-TD patients, and in patients with co-mutations of the WT1 gene (data not shown). AlloSCT performed in CR1 in 11 pts (21%) did not improve outcome. In multivariable analyses, the presence of UBTF-TD was associated with a trend for shorter OS (HR: 1.39; 95% CI 0.98-2, p=.06) and significantly worse EFS (HR: 1.68; 95% CI 1.24-2.3; p<.001).
Conclusions: This analysis of a large cohort of adult patients with newly diagnosed AML revealed that UBTFmutations can be found in about 1% of all patients with AML, but are considerably more common in young adults. As in pediatric patients, they are associated with a specific cytogenetic and molecular profile, i.e. high rate of +8, FLT3-ITD and WT1 mutations. As a novel finding, adult UBTF-TD patients frequently showed signs of myelodysplasia. The distinct molecular genotype and the fact that UBTF-TDs were associated with a dismal outcome, even in patients treated with alloSCT in CR1, support the classification of this group as a new disease entity and indicate that novel treatment strategies are needed in this subgroup of patients.
Disclosures
Krug:, Leo Pharma,: Honoraria; BMS: Honoraria; , Sanofi: Honoraria; AbbVie: Honoraria. Sauer:Pfizer: Honoraria; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Ridgeline Discoveries: Membership on an entity's Board of Directors or advisory committees. Hochhaus:BMS: Research Funding; Pfizer: Research Funding; Incyte: Research Funding; Novartis: Research Funding. Steffen:Jazz Pharmaceuticals: Other: Travel/Congress Participation Support; AbbVie: Other: Travel/Congress Participation Support. Einsele:BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants; Sanofi: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Other: travel grants. Burchert:Incyte: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; AOP Health: Honoraria, Research Funding. Schliemann:Philogen S.p.A.: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Other: travel grants; Astellas: Consultancy; Astrazeneca: Consultancy; Boehringer-Ingelheim: Research Funding; BMS: Consultancy, Other: travel grants; Jazz: Consultancy, Research Funding; Novartis: Consultancy; Roche: Consultancy; Pfizer: Consultancy. Krause:Art-tempi: Honoraria; Kosmas: Honoraria; Abbvie: Other: Expenses. Haenel:Gilead: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; JAZZ: Consultancy, Honoraria; Pfizer: Honoraria; Takeda: Consultancy, Honoraria. Platzbecker:Takeda: Honoraria; Novartis: Honoraria; Silence Therapeutics: Honoraria; Janssen: Honoraria; Geron: Honoraria; BMS/Celgene: Honoraria; Abbvie: Honoraria; Jazz: Honoraria. Ehninger:AvenCell Europe GmbH: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Support for meeting attendance. Schetelig:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Thiede:Kronos Bio, Inc.: Honoraria; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen Pharmaceuticals: Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AgenDix GmbH: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.